On March 13, 2020, President Donald Trump declared a national emergency under the National Emergencies Act and made an emergency determination under the Stafford Act. This announcement follows the January 31, 2020 declaration of a public health emergency under the Public Health Service Act by the Secretary of the US Department of Health and Human Services (HHS).
When the President declares a disaster or emergency under the Stafford Act or National Emergencies Act and the HHS Secretary declares public health, the Secretary is authorized to take certain actions in addition to his regular authorities. Under section 1135 of the Social Security Act, he may temporarily waive or modify certain Medicare, Medicaid, and Children’s Health Insurance Program (CHIP) requirements to ensure that sufficient health care items and services are available to meet the needs of individuals and that providers who provide such services in good faith can be reimbursed and exempted from sanctions. Shortly after the presidential declaration on March 13, the Centers for Medicare and Medicaid Services (CMS) announced a set of blanket waivers specific to the COVID-19 pandemic (COVID-19 waivers).
“Blanket” Medicare Waiver Authority
Once an 1135 Waiver is authorized, in past emergencies such as a localized natural disaster, health care providers have submitted requests to operate under that authority to the State Survey Agency or CMS Regional Office. The requests generally have included a justification for the waiver and the expected duration of the modification requested. In pandemic situations, like COVID-19, where all similarly situated providers in an emergency area need a waiver or modification, CMS can implement specific waivers or modifications under the 1135 authority on a “blanket” basis. The decision to implement a “blanket” waiver or modification of a Medicare, Medicaid, or CHIP requirement is based on the need and frequency of requests for specific waivers or modifications in response to the disaster or emergency.
Given the anticipated impact that COVID-19 will have on hospitals across the country, CMS has authorized several blanket waivers of Medicare provisions that typically impose day limits and length of stays on hospitals, skilled nursing facilities, and inpatient rehabilitation facilities. These waivers will enable patients to be relocated to different care settings in order to increase capacity for hospitals and other providers on the frontline of the outbreak and ensure that providers get reimbursed for providing services during this uncertain time. Medicare waivers included in the COVID-19 blanket authority include:
- Skilled Nursing Facilities: Waiving 3-day prior hospitalization rule for coverage of a skilled nursing facility (SNF) stay
- Critical Access Hospital: Waiving 25-bed requirement to classify as a critical access hospital and that the length of stay be limited to 96 hours
- Acute Care Hospital: Allowing acute care hospitals to house acute-care inpatients in excluded distinct part units, where the distinct part unit’s beds are appropriate for acute care inpatient
- Inpatient Rehabilitation Facility: Waiving the 60-percent rule for inpatient rehabilitation facilities
- Long-Term Acute Care Hospitals: Permitting long-term care hospitals to exclude patient stays from the 25-day average length requirement if the admit or discharge is ordered due to the emergency
- Durable Medical Equipment: Relaxing standards for lost, destroyed, irreparably damaged or otherwise unusable durable medical equipment, prosthetics, orthopedics, and supplies
In addition to Medicare waivers and flexibilities, CMS is also modifying provider enrollment requirements to ensure patients have access to providers during the national emergency, including suspending certain Medicare enrollment screening requirements, waiving requirements licensure requirements for out-of-state providers, and implementing streamlined application processes.
Medicaid Waiver Authority
The national emergency declaration enables CMS to grant state and territorial Medicaid agencies a wider range of flexibilities under section 1135 waivers. The objective of these waivers is to provide states and providers with the flexibility to address capacity issues and to relieve the administrative burden associated with the usual claims processing and credentialing of providers. Unlike the Medicare blanket waivers, the COVID-19 waivers applicable to providers and services governed by Medicaid laws require states to individually apply for relief. CMS highlighted the following examples of Medicaid waivers available under section 1135 of the Act:
- Prior Authorization: Waiving prior authorization requirements in fee-for-service programs
- Out-of-State Providers: Permitting providers located out-of-state or -territory to provide care to another state’s Medicaid enrollees affected by the emergency (referring to the ability to receive payment)
- Provider Enrollment: Suspending certain provider enrollment and revalidation requirements to increase access to care
- Equivalent Licensure: Waiving requirements that physicians and other healthcare professionals be licensed in the state in which they are providing services, as long as they have equivalent licensure in another state
- Provision of Services at Alternative Settings: Allowing (unlicensed) alternative facilities (e.g., nursing homes, intermediate care facilities) to be reimbursed at facility rates
- Skilled Nursing Facility Admission Screening and Annual Review Assessments: Suspending requirements for certain pre-admission and annual screenings for nursing home residents.
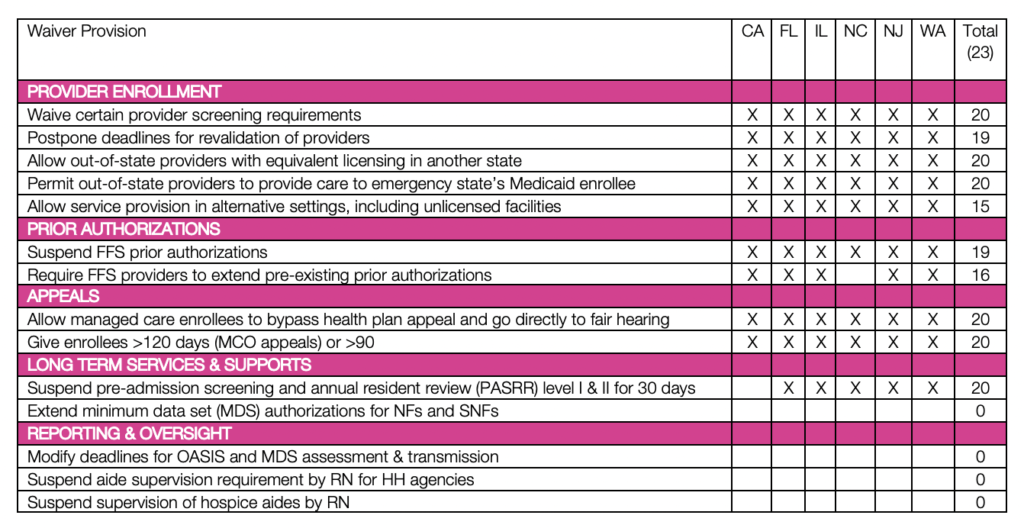
On March 22, 2020, CMS released an 1135 Medicaid & CHIP Checklist to assist states in applying for an 1135 waiver. This checklist streamlines the waiver application process for states and CMS. The checklist is pre-populated with relevant and commonly requested 1135 authorities under the following categories: 1) Medicaid Authorizations, 2) Long-Term Services and Supports, 3) Fair Hearings, 4) Provider Enrollment, 5) Reporting and Oversight. Additionally, states are permitted to request waivers of provisions that are not included on the checklist; only 3 states have had additional requests approved as of March 25, 2020.
Section 1135 waivers are effective March 1, 2020, and will end upon termination of the public health emergency, including any extensions. As of March 25, 2020, CMS has approved 1135 waivers for 23 states. The table below provides a summary of approved waivers provisions for select states, including states like Washington and California that have experienced some of the more extensive outbreaks of COVID-19 to date. Updated tracking of state waivers can be viewed on the Kaiser Family Foundation Medicaid Emergency Authority Tracker: Approved State Actions to Address COVID-19.
In our ongoing effort to help our partners navigate this crisis, Atrómitos will continue to review and assess federal Medicare and Medicaid policy changes related to COVID-19. With combined decades working in state and federal government, Atrómitos has the experience and expertise to support your organization’s policy and regulatory needs. If you are interested in understanding regulatory issues specific to your market (local and service sector), we are ready and willing to dive in and identify, track, and analyze legislative and administrative changes that may impact your business and programs.


